Hanna HI1053B is a glass body, refillable, double junction pH electrode with a BNC connector. This electrode has three ceramic junctions in the outer reference cell for an increased flow rate of reference electrolyte and a conical pH sensing tip that is made with low temperature glass.
These design considerations are ideal for emulsions, fats and creams, soil and semi-solid samples, low conductivity solutions, and for measuring samples at cooler temperatures.
- Triple Ceramic Junction
- Double Junction Design
- Refillable Electrode
Details
Hanna Instruments offers a wide variety of pH electrodes that are designed for many different applications. The type of glass used for sensing pH, bulb shape, body material, type of junction, type of reference and electrolyte used are just some of the design considerations.
The HI1053B uses low temperature (LT) glass, conical bulb, glass body, triple ceramic junction and is refillable with 3.5M KCl.
Low Temperature Glass Formulation
A standard pH electrode uses general purpose (GP) glass that has a resistance of 100 megaohms at 25°C. The HI1053B uses LT (low temperature) glass that has a resistance of around 50 megaohms at 25°C. As the temperature of the glass decreases in the sample, the resistance of the LT glass will approach that of GP glass. If using GP glass, the resistance would increase above the optimum range, resulting in increased impedance and ultimately affecting the measurement. The HI1053B is suitable to use with samples that measure from -5 to 100°C.
Conical Glass Tip
The conical shaped tip design allows for penetration into solids, semi solids, and emulsions for the direct measurement of pH in food products, soil, and emulsions like hand creams.
Glass Body
The glass body is ideal for laboratory use. The glass is resistant to many harsh chemicals and is easily cleaned. The glass body also allows for a fast transfer of heat to the internal reference electrolyte. The mV generated by the reference cell is temperature dependent. The faster the electrode reaches equilibrium, the steadier the reference potential.
Triple Ceramic Junction
The triple ceramic junction allows a higher flow rate of electrolyte from the reference cell into the solution. A standard pH electrode will use a single ceramic junction that allows for 15 to 20 µL/hour of electrolyte to flow; the HI1053B has three ceramic junctions, providing for 40 to 50 µL/hour of electrolyte to flow. This high flow rate provides faster electrode response and a more stable measurement in viscous solutions or samples of low conductivity.
Double Junction Reference
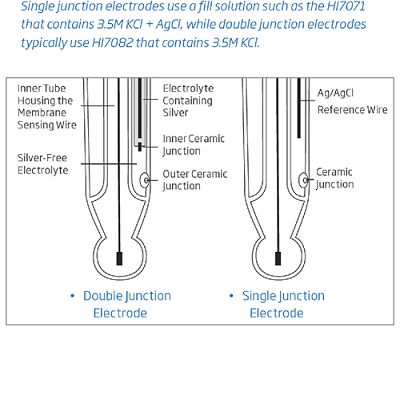
A double junction electrode has an internal compartment surrounding the reference wire. Silver ions are present in the electrolyte of the internal compartment, which houses the Ag/AgCl reference wire; the electrolyte outside this compartment is silver free. The double junction design means that virtually no silver from the electrode enters the sample. This design allows measurement in applications where silver ions in the sample are undesirable or for samples that contains sulfides that can cause the silver to precipitate and clog the junction. Clogging of the junction will result in drifty and erratic readings.
Refillable
The HI1053B is a refillable probe. Since it is a double junction pH electrode, the fill solution is the HI7082 3.5M KCl. This solution does not contain any silver which is necessary for single junction electrode fill solutions.
BNC Connector
The HI1053B uses a BNC connector. This type of connector is universal in that it can be used on any pH meter that has the female BNC probe input. Other type of connectors include DIN, screw type, T-type, and 3.5mm to name a few. These types of connectors tend to be proprietary for a particular type of meter and are not interchangeable.
Conventional electrodes are normally single junction. As depicted by the figure above, these electrodes have only a single junction between the internal refernce wire and the external solution. Under adverse conditions, such as high pressure, high temperature, highly acidic or alkaline solutions, the positive flow of the electrolyte through the junction is often reversed resulting in the ingress of sample solution into the reference compartment. If this is left unchecked, the reference electrode can become contaminated, leading to complete electrode failure. Another potential problem with single junction electrodes is the clogging of the junction due to silver chloride (AgCl) precipitation. Silver can be easily precipitate in samples that contain Tris buffer, sulfides or heavy metals. When the electrolyte solution makes contact with the sample, some AgCl will precipitate on the external face of the junction. The result is drifty readings obtained from the sensor.
Hanna’s double junction system, as the name implies, has two junctions, only one of which is in contact with the sample as shown in the figure. Under adverse conditions, the same tendency of sample ingress is evident. However, as the reference electrode system is separated physically from the intermediate electrolyte area, the contamination of the electrode is minimized. The likelihood of clogging of the junction is also reduced with a double junction electrode since the outer reference cell uses a fill solution that is “silver-free”. Since there is no silver present, there is no precipitate forming to clog the junction.
Specifications
| Body Material | glass |
|---|---|
| Reference | double, Ag/AgCl |
| Junction / Flow Rate | ceramic, triple / 40-50 μL/h |
| Electrolyte | 3.5M KCl |
| Range | pH: 0 to 12 |
| Max Pressure | 0.1 bar |
| Tip Shape | conic (12 x 12 mm) |
| Diameter | 12 mm |
| Body Length / Overall Length | 120 mm / 175.5 mm |
| Recommended Operating Temperature | -5 to 70°C (23 to 158°F) |
| Temperature Sensor | no |
| Matching Pin | no |
| Amplifier | no |
| Digital | no |
| Cable | coaxial; 1 m (3.3’) |
| Connection | BNC |
| Applications | emulsions, fats and creams, low conductivity solutions, potable water, semi-solid products, soil samples |